

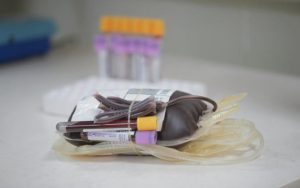
Since April 2018, Nucleic Acid Testing (NAT) using Polymerase Chain Reaction (PCR) technology is being used for identifying Hepatitis B and Hepatitis C viruses on every single donor in addition to CLIA which was already being performed. Thus fulfilling FDA (USA) requirement for donor screening.


Fulfill your Islamic obligation of Zakat with Indus Development Foundation


Transform lives with your Zakat. A gift of healing and hope for those in need.


Make a difference through Sadaqah. Your contributions empower positive change in healthcare.


Fuel the expansion of Healthcare Facilities. Help us build the largest private hospital in Pakistan.


NourishMom for a healthy start. Your donation supports essential nutrition for mothers during pregnancy and post-delivery.


Champion Safe Births for all mothers. Contribute to maternal wellness for a healthier tomorrow.
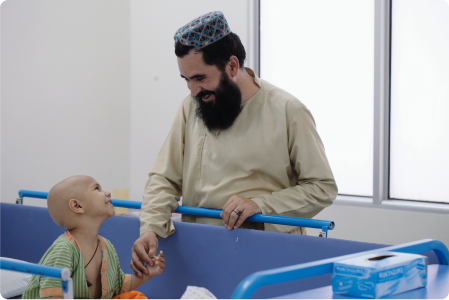

Join the fight against Childhood Cancer. Your generosity fosters strength in our young warriors.


Give the gift of Primary Healthcare. Your donation ensures essential medical services for all.


Support any cause with your General Donations. Every contribution adds up to create a healthier future.

